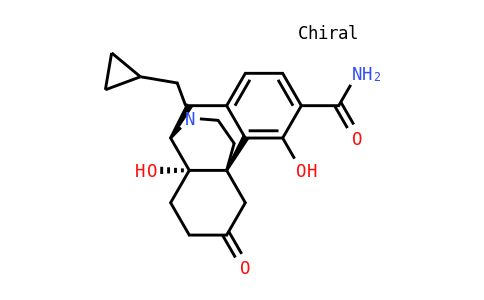
Samidorphan
Samidorphan, also known as ALKS-33 and RDC-0313, is an opioid modulator with μ-antagonist properties. It is under development by Alkermes for the treatment of major depressive disorder and possibly other psychiatric conditions.
仅供研究使用。 我们不向患者出售。
化学信息
| 名称 | Samidorphan |
|---|---|
| 同义词 | Samidorphan; RDC 0313; RDC-0313; RDC0313; ALKS-33; ALKS33; ALKS 33; |
| 英文同义词 | Samidorphan; RDC 0313; RDC-0313; RDC0313; ALKS-33; ALKS33; ALKS 33; |
| 分子式 | C21H26N2O4 |
| 分子量 | 370.44 |
| Smile | O=C(C(C(O)=C1[C@@]23C4)=CC=C1C[C@@H](N(CC5CC5)CC3)[C@]2(O)CCC4=O)N |
| InChiKey | RYIDHLJADOKWFM-MAODMQOUSA-N |
| InChi | InChI=1S/C21H26N2O4/c22-19(26)15-4-3-13-9-16-21(27)6-5-14(24)10-20(21,17(13)18(15)25)7-8-23(16)11-12-1-2-12/h3-4,12,16,25,27H,1-2,5-11H2,(H2,22,26)/t16-,20-,21-/m1/s1 |
| Cas号 | 852626-89-2 |
| 相关CAS号 |
订购信息
| 包装 | 价格 | 库存 | 纯度 | 备货期 |
|---|---|---|---|---|
| 大货 | 询价 | 询价 | 询价 |
| 外观性状 | 固体粉末 |
|---|---|
| 纯度 | 98% Min. |
| 存储 | 短期(几天到几周)为0-4摄氏度,长期(几个月)为-20摄氏度 |
| 可溶性 | 溶于DMSO |
| 处理方式 | |
| 运输条件 | 作为非危险化学品在环境温度下装运。这种产品在正常运输和海关工作期间可以稳定几周. |
| 海关编码 |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Shram MJ, Silverman B, Ehrich E, Sellers EM, Turncliff R. Use of Remifentanil in a Novel Clinical Paradigm to Characterize Onset and Duration of Opioid Blockade by Samidorphan, a Potent μ-Receptor Antagonist. J Clin Psychopharmacol. 2015 Jun;35(3):242-9. doi: 10.1097/JCP.0000000000000320. PubMed PMID: 25928699; PubMed Central PMCID: PMC4415969.
2: Turncliff R, DiPetrillo L, Silverman B, Ehrich E. Single- and multiple-dose pharmacokinetics of samidorphan, a novel opioid antagonist, in healthy volunteers. Clin Ther. 2015 Feb 1;37(2):338-48. doi: 10.1016/j.clinthera.2014.10.001. Epub 2014 Oct 29. PubMed PMID: 25456560.
3: Ehrich E, Turncliff R, Du Y, Leigh-Pemberton R, Fernandez E, Jones R, Fava M. Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology. 2015 May;40(6):1448-55. doi: 10.1038/npp.2014.330. Epub 2014 Dec 18. PubMed PMID: 25518754; PubMed Central PMCID: PMC4397403.
4: Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M, Du Y, Leigh-Pemberton R, DiPetrillo L, Silverman B, Ehrich E. Opioid Modulation With Buprenorphine/Samidorphan as Adjunctive Treatment for Inadequate Response to Antidepressants: A Randomized Double-Blind Placebo-Controlled Trial. Am J Psychiatry. 2016 May 1;173(5):499-508. doi: 10.1176/appi.ajp.2015.15070921. Epub 2016 Feb 12. PubMed PMID: 26869247.
5: O'Malley SS, Todtenkopf MS, Du Y, Ehrich E, Silverman BL. Effects of the Opioid System Modulator, Samidorphan, on Measures of Alcohol Consumption and Patient Reported Outcomes in Adults with Alcohol Dependence. Alcohol Clin Exp Res. 2018 Jul 28. doi: 10.1111/acer.13849. [Epub ahead of print] PubMed PMID: 30055046.
6: Ragguett RM, Rong C, Rosenblat JD, Ho RC, McIntyre RS. Pharmacodynamic and pharmacokinetic evaluation of buprenorphine + samidorphan for the treatment of major depressive disorder. Expert Opin Drug Metab Toxicol. 2018 Apr;14(4):475-482. doi: 10.1080/17425255.2018.1459564. Epub 2018 Apr 6. Review. PubMed PMID: 29621905.
7: Silverman BL, Martin W, Memisoglu A, DiPetrillo L, Correll CU, Kane JM. A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of olanzapine-induced weight gain in healthy volunteers. Schizophr Res. 2018 May;195:245-251. doi: 10.1016/j.schres.2017.10.014. Epub 2017 Nov 20. PubMed PMID: 29158012.
8: Sun L, McDonnell D, Liu J, von Moltke L. Bioequivalence of Olanzapine Given in Combination With Samidorphan as a Bilayer Tablet (ALKS 3831) Compared With Olanzapine-Alone Tablets: Results From a Randomized, Crossover Relative Bioavailability Study. Clin Pharmacol Drug Dev. 2018 Jul 30. doi: 10.1002/cpdd.601. [Epub ahead of print] PubMed PMID: 30059196.