1: Nirogi R, Shinde A, Goyal VK, Ravula J, Benade V, Jetta S, Pandey SK, Subramanian R, Chowdary Palacharla VR, Mohammed AR, Abraham R, Dogiparti DK, Kalaikadhiban I, Jayarajan P, Jasti V, Bogan RK. Samelisant (SUVN-G3031), a histamine 3 receptor inverse agonist: Results from the phase 2 double-blind randomized placebo-controlled study for the treatment of excessive daytime sleepiness in adult patients with narcolepsy. Sleep Med. 2024 Oct 30;124:618-626. doi: 10.1016/j.sleep.2024.10.037. Epub ahead of print. PMID: 39504585.
2: Nirogi R, Benade V, Daripelli S, Subramanian R, Kamuju V, Bhyrapuneni G, Muddana NR, Mekala VR, Petlu S, Jayarajan P, Badange R, Shinde A, Jasti V. Samelisant (SUVN-G3031), a potent, selective and orally active histamine H3 receptor inverse agonist for the potential treatment of narcolepsy: pharmacological and neurochemical characterisation. Psychopharmacology (Berl). 2021 Jun;238(6):1495-1511. doi: 10.1007/s00213-021-05779-x. Epub 2021 Feb 7. PMID: 33550481.
3: Nirogi R, Grandhi VR, Medapati RB, Ganuga N, Benade V, Gandipudi S, Manoharan A, Abraham R, Jayarajan P, Bhyrapuneni G, Shinde A, Badange RK, Subramanian R, Petlu S, Jasti V. Histamine 3 receptor inverse agonist Samelisant (SUVN-G3031): Pharmacological characterization of an investigational agent for the treatment of cognitive disorders. J Psychopharmacol. 2021 Jun;35(6):713-729. doi: 10.1177/0269881120986418. Epub 2021 Feb 5. PMID: 33546570.
4: Nirogi R, Bhyrapuneni G, Muddana NR, Manoharan A, Shinde AK, Mohammed AR, Padala NP, Ajjala DR, Subramanian R, Palacharla VRC. Absorption, distribution, metabolism, excretion (ADME), drug-drug interaction potential and prediction of human pharmacokinetics of SUVN-G3031, a novel histamine 3 receptor (H3R) inverse agonist in clinical development for the treatment of narcolepsy. Eur J Pharm Sci. 2020 Sep 1;152:105425. doi: 10.1016/j.ejps.2020.105425. Epub 2020 Jun 10. PMID: 32534194.
5: Nirogi R, Mudigonda K, Bhyrapuneni G, Muddana NR, Shinde A, Goyal VK, Pandey SK, Mohammed AR, Ravula J, Jetta S, Palacharla VRC. Safety, Tolerability, and Pharmacokinetics of SUVN-G3031, a Novel Histamine-3 Receptor Inverse Agonist for the Treatment of Narcolepsy, in Healthy Human Subjects Following Single and Multiple Oral Doses. Clin Drug Investig. 2020 Jul;40(7):603-615. doi: 10.1007/s40261-020-00920-8. PMID: 32399853.
6: Nirogi R, Ajjala DR, Prakash Padala NS, Kalaikadhiban I, Rayapati LP, Chunduru P, Shinde A. LC-MS/MS method for the quantification of SUVN-G3031, a novel H3 receptor inverse agonist for narcolepsy treatment. Bioanalysis. 2020 Apr;12(8):533-544. doi: 10.4155/bio-2020-0020. Epub 2020 Apr 30. PMID: 32351118.
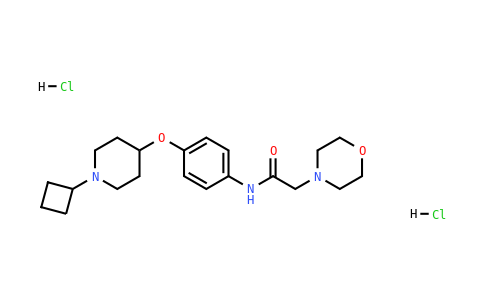
7: Nirogi R, Shinde A, Mohammed AR, Badange RK, Reballi V, Bandyala TR, Saraf SK, Bojja K, Manchineella S, Achanta PK, Kandukuri KK, Subramanian R, Benade V, Palacharla RC, Jayarajan P, Pandey S, Jasti V. Discovery and Development of N-[4-(1-Cyclobutylpiperidin-4-yloxy)phenyl]-2-(morpholin-4-yl)acetamide Dihydrochloride (SUVN-G3031): A Novel, Potent, Selective, and Orally Active Histamine H3 Receptor Inverse Agonist with Robust Wake-Promoting Activity. J Med Chem. 2019 Feb 14;62(3):1203-1217. doi: 10.1021/acs.jmedchem.8b01280. Epub 2019 Jan 25. PMID: 30629436.
8: Mason VL. Alzheimer's Association International Conference on Alzheimer's Disease 2015 (AAIC 2015) (July 18-23, 2015 - Washington, D.C., USA). Drugs Today (Barc). 2015 Jul;51(7):447-52. doi: 10.1358/dot.2015.51.7.2375989. PMID: 26261847.