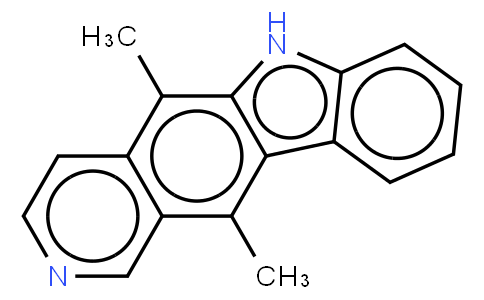
Ellipticine
编号: 16122811
Cas号: 519-23-3
纯度: 98%
Ellipticine is a DNA intercalating agent and a DNA topoisomerase II inhibitor. Ellipticine is also a natural product, isolated in 1959 from the Australian evergreen tree of the Apocynaceae family. Ellipticine was found to be an extremely promising anticancer drug. The planar polycyclic structure was found to interact with DNA through intercalation, exhibiting a high DNA binding affinity (10(6) M(-1)). The presence of protonatable ring nitrogens distinguished ellipticine from other simple intercalators. Both monocationic and uncharged species were found to be present under physiological conditions. The positive charge stabilized the binding of ellipticine to nucleic acids, while the more lipophilic uncharged compound was shown to readily penetrate membrane barriers. The structural nature of these compounds offers a plausible basis for the implication of multiple modes of action, including DNA binding, interactions with membrane barriers, oxidative bioactivation and modification of enzyme function; most notably that of topoisomerase II and telomerase. ( Curr Med Chem Anticancer Agents. 2004 Mar;4(2):149-72 ).
仅供研究使用。 我们不向患者出售。
化学信息
| 名称 | Ellipticine |
|---|---|
| Iupac 化学名称 | Ellipticine |
| 同义词 | CCG-36483; CCG36483; CCG 36483; BRN-0221300; BRN0221300; BRN 0221300; DB-052047; K00071; LP00531; LS-133282; NSC 71795; NSC71795; NSC-71795; |
| 英文同义词 | CCG-36483; CCG36483; CCG 36483; BRN-0221300; BRN0221300; BRN 0221300; DB-052047; K00071; LP00531; LS-133282; NSC 71795; NSC71795; NSC-71795; |
| 分子式 | C17H14N2 |
| 分子量 | 246.31 |
| Smile | Cc1c2ccncc2c(c3c1[nH]c4c3cccc4)C |
| InChiKey | CTSPAMFJBXKSOY-UHFFFAOYSA-N |
| InChi | InChI=1S/C17H14N2/c1-10-14-9-18-8-7-12(14)11(2)17-16(10)13-5-3-4-6-15(13)19-17/h3-9,19H,1-2H3 |
| Cas号 | 519-23-3 |
| MDL | MFCD00010524 |
| 相关CAS号 |
订购信息
| 包装 | 价格 | 库存 | 纯度 | 备货期 |
|---|---|---|---|---|
| 大货 | 询价 | 询价 | 询价 |