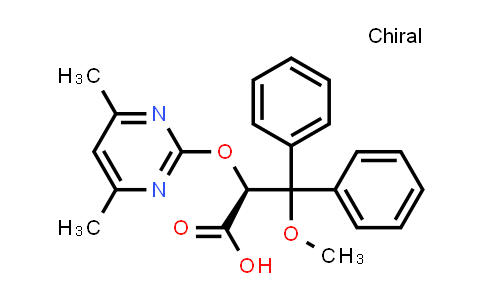
Ambrisentan
Ambrisentan, also known as BSF-208075 and LU-208075, is a drug indicated for use in the treatment of pulmonary hypertension. Ambrisentan, which relaxes those muscles, is an endothelin receptor antagonist, and is selective for the type A endothelin receptor (ETA). Ambrisentan significantly improved exercise capacity (6-minute walk distance) compared with placebo in two double-blind, multicenter trials (ARIES-1 and ARIES-2). Ambrisentan was approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency, and designated an orphan drug, for the treatment of pulmonary hypertension.
仅供研究使用。 我们不向患者出售。
化学信息
| 名称 | Ambrisentan |
|---|---|
| Iupac 化学名称 | (S)-2-((4,6-dimethylpyrimidin-2-yl)oxy)-3-methoxy-3,3-diphenylpropanoic acid |
| 同义词 | BSF-208075; BSF208075; BSF 208075; LU-208075; LU208075; LU 208075; Ambrisentan. trade name Letairis; Volibris; pulmonext. |
| 英文同义词 | BSF-208075; BSF208075; BSF 208075; LU-208075; LU208075; LU 208075; Ambrisentan. trade name Letairis; Volibris; pulmonext. |
| 分子式 | C22H22N2O4 |
| 分子量 | 378.428 |
| Smile | O=C(O)[C@@H](OC1=NC(C)=CC(C)=N1)C(C2=CC=CC=C2)(OC)C3=CC=CC=C3 |
| InChiKey | OUJTZYPIHDYQMC-LJQANCHMSA-N |
| InChi | InChI=1S/C22H22N2O4/c1-15-14-16(2)24-21(23-15)28-19(20(25)26)22(27-3,17-10-6-4-7-11-17)18-12-8-5-9-13-18/h4-14,19H,1-3H3,(H,25,26)/t19-/m1/s1 |
| Cas号 | 177036-94-1 |
| 相关CAS号 |
订购信息
| 包装 | 价格 | 库存 | 纯度 | 备货期 |
|---|---|---|---|---|
| 大货 | 询价 | 询价 | 询价 |
| 外观性状 | 固体粉末 |
|---|---|
| 纯度 | 98% Min. |
| 存储 | 干燥、黑暗,短期(日至周)在0-4摄氏度,长期(月至年)在-20摄氏度。 |
| 可溶性 | 可溶于DMSO |
| 处理方式 | |
| 运输条件 | 作为非危险化学品在环境温度下装运。这种产品在正常运输和海关工作期间可以稳定几周。 |
| 海关编码 |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Vachiéry JL, Hoeper MM, Peacock AJ, Sitbon O, Cheli M, Church C, Olsson KM, Palazzini M, Waterhouse B, Langley J, Galié N. Ambrisentan use for pulmonary arterial hypertension in a post-authorization drug registry: The VOLibris Tracking Study. J Heart Lung Transplant. 2016 May 6. pii: S1053-2498(16)30117-6. doi: 10.1016/j.healun.2016.04.013. [Epub ahead of print] PubMed PMID: 27282418.
2: Cedars AM, Saef J, Peterson LR, Coggan AR, Novak EL, Kemp D, Ludbrook PA. Effect of Ambrisentan on Exercise Capacity in Adult Patients After the Fontan Procedure. Am J Cardiol. 2016 May 1;117(9):1524-32. doi: 10.1016/j.amjcard.2016.02.024. Epub 2016 Feb 23. PubMed PMID: 27063478.
3: Takeuchi T, Sakao S, Kato F, Naito A, Jujo T, Yasuda T, Tanabe N, Tatsumi K. Pulmonary haemodynamics are correlated with intimal lesions in a rat model of severe PAH: attenuation of pulmonary vascular remodelling with ambrisentan. Histol Histopathol. 2016 Apr 6:11764. [Epub ahead of print] PubMed PMID: 27048555.
4: Hakamata A, Odagiri K, Miyakawa S, Irisawa H, Takeuchi K, Inui N, Tanaka S, Uchida S, Watanabe H. Pharmacokinetic and Pharmacodynamic Comparison of Sildenafil-Bosentan and Sildenafil-Ambrisentan Combination Therapies for Pulmonary Hypertension. Clin Transl Sci. 2016 Feb;9(1):29-35. doi: 10.1111/cts.12382. Epub 2016 Jan 12. PubMed PMID: 26756977.
5: Ambrisentan (Letairis) and tadalafil (Adcirca) for pulmonary arterial hypertension. Med Lett Drugs Ther. 2016 Jan 4;58(1485):2-4. PubMed PMID: 26714240.
6: Enderle Y, Meid AD, Friedrich J, Grünig E, Wilkens H, Haefeli WE, Burhenne J. Dried Blood Spot Technique for the Monitoring of Ambrisentan, Bosentan, Sildenafil, and Tadalafil in Patients with Pulmonary Arterial Hypertension. Anal Chem. 2015 Dec 15;87(24):12112-20. doi: 10.1021/acs.analchem.5b03077. Epub 2015 Dec 3. PubMed PMID: 26583764.
7: Hassoun PM, Zamanian RT, Damico R, Lechtzin N, Khair R, Kolb TM, Tedford RJ, Hulme OL, Housten T, Pisanello C, Sato T, Pullins EH, Corona-Villalobos CP, Zimmerman SL, Gashouta MA, Minai OA, Torres F, Girgis RE, Chin K, Mathai SC. Ambrisentan and Tadalafil Up-front Combination Therapy in Scleroderma-associated Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2015 Nov 1;192(9):1102-10. doi: 10.1164/rccm.201507-1398OC. PubMed PMID: 26360334; PubMed Central PMCID: PMC4642204.
8: Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grünig E, Oudiz RJ, Vonk-Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JH, Langley J, Rubin LJ; AMBITION Investigators. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med. 2015 Aug 27;373(9):834-44. doi: 10.1056/NEJMoa1413687. PubMed PMID: 26308684.
9: Patel JK, Patel NK. Stability-Indicating RP-HPLC Method for the Determination of Ambrisentan and Tadalafil in Pharmaceutical Dosage Form. Sci Pharm. 2014 May 22;82(4):749-63. doi: 10.3797/scipharm.1403-22. eCollection 2014 Dec. PubMed PMID: 26279975; PubMed Central PMCID: PMC4500576.
10: Peacock AJ, Zamboni W, Vizza CD. Ambrisentan for the treatment of adults with pulmonary arterial hypertension: a review. Curr Med Res Opin. 2015;31(9):1793-807. doi: 10.1185/03007995.2015.1074890. Epub 2015 Aug 27. Review. PubMed PMID: 26196225.
11: Muraoka H, Imamura T, Hatano M, Maki H, Yao A, Kinugawa K, Komuro I. Secure Combination Therapy With Low-Dose Bosentan and Ambrisentan to Treat Portopulmonary Hypertension Minimizing Each Adverse Effect. Int Heart J. 2015;56(4):471-3. doi: 10.1536/ihj.15-007. Epub 2015 Jun 18. PubMed PMID: 26084462.
12: Bose N, Bena J, Chatterjee S. Evaluation of the effect of ambrisentan on digital microvascular flow in patients with systemic sclerosis using laser Doppler perfusion imaging: a 12-week randomized double-blind placebo controlled trial. Arthritis Res Ther. 2015 Mar 5;17:44. doi: 10.1186/s13075-015-0558-9. PubMed PMID: 25876611; PubMed Central PMCID: PMC4384235.
13: Li XQ, Li YJ, Wang Y. Ambrisentan May Improve Exercise Tolerance and Cardiac Function in Patients With Pulmonary Hypertension. Clin Ther. 2015 Jun 1;37(6):1270-9. doi: 10.1016/j.clinthera.2015.03.011. Epub 2015 Apr 7. Review. PubMed PMID: 25862136.
14: Yamashita Y, Tsujino I, Sato T, Yamada A, Watanabe T, Ohira H, Nishimura M. Hemodynamic effects of ambrisentan-tadalafil combination therapy on progressive portopulmonary hypertension. World J Hepatol. 2014 Nov 27;6(11):825-9. doi: 10.4254/wjh.v6.i11.825. PubMed PMID: 25429321; PubMed Central PMCID: PMC4243157.
15: Markert C, Kastner IM, Hellwig R, Kalafut P, Schweizer Y, Hoffmann MM, Burhenne J, Weiss J, Mikus G, Haefeli WE. The effect of induction of CYP3A4 by St John's wort on ambrisentan plasma pharmacokinetics in volunteers of known CYP2C19 genotype. Basic Clin Pharmacol Toxicol. 2015 May;116(5):423-8. doi: 10.1111/bcpt.12332. Epub 2014 Nov 8. PubMed PMID: 25286744.
16: Zhang L, Zou Y, Song SH, Wang ZX, Ding GS, Ma J, Ni ZJ, Fu H, Shi XM, Gao XG, Han QC, Guo WY, Fu ZR. Ambrisentan improves the outcome of rats with liver transplantation partially through reducing nephrotoxicity. Eur Rev Med Pharmacol Sci. 2014;18(17):2575-83. PubMed PMID: 25268107.
17: Markert C, Wirsching T, Hellwig R, Burhenne J, Weiss J, Riedel KD, Mikus G, Haefeli WE. Lack of a clinically significant interaction of grapefruit juice with ambrisentan and bosentan in healthy adults. Int J Clin Pharmacol Ther. 2014 Nov;52(11):957-64. doi: 10.5414/CP202164. PubMed PMID: 25207548.
18: Wen L, Jiang X, An P, He J, Zheng L, Liu Q, Peng F, Xu X, Jing Z. [Long-term effects with ambrisentan monotherapy in patients with pulmonary arterial hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2014 Jun;42(6):469-73. Chinese. PubMed PMID: 25164219.
19: Chung L, Ball K, Yaqub A, Lingala B, Fiorentino D. Effect of the endothelin type A-selective endothelin receptor antagonist ambrisentan on digital ulcers in patients with systemic sclerosis: results of a prospective pilot study. J Am Acad Dermatol. 2014 Aug;71(2):400-1. doi: 10.1016/j.jaad.2014.04.028. PubMed PMID: 25037794; PubMed Central PMCID: PMC4356009.
20: Condliffe R, Elliot CA, Hurdman J, Sabroe I, Billings C, Kiely DG, Hamilton N. Ambrisentan therapy in pulmonary hypertension: clinical use and tolerability in a referral centre. Ther Adv Respir Dis. 2014 Apr 30;8(3):71-77. [Epub ahead of print] PubMed PMID: 24787237.