VU0119498
VU0119498 is a M1 muscarinic receptor agonist (EC50 = 3.1 μM) and pan mAChR M3, M5 positive allosteric modulator (PAM). VU0119498 is a neuroprotective agent.
For research use only. We do not sell to patients.
Chemical Information
| Name | VU0119498 |
|---|---|
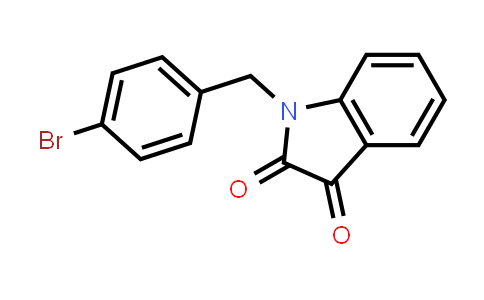
| Iupac Chemical Name | 1-[(4-bromophenyl)methyl]indole-2,3-dione |
| Synonyms | VU0119498, VU 0119498, VU-0119498 |
| Molecular Formula | C15H10BrNO2 |
| Molecular Weight | 316.15 |
| Smile | O=C1N(CC2=CC=C(Br)C=C2)C3=C(C=CC=C3)C1=O |
| InChiKey | DELLOEULSHGYCG-UHFFFAOYSA-N |
| InChi | InChI=1S/C15H10BrNO2/c16-11-7-5-10(6-8-11)9-17-13-4-2-1-3-12(13)14(18)15(17)19/h1-8H,9H2 |
| CAS Number | 79183-37-2 |
| Related CAS |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | Solid powder |
|---|---|
| Purity | 98% Min. |
| Storage | Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
| Solubility | Soluble in DMSO |
| Handling | |
| Shipping Condition | Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs. |
| HS Code |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Bridges TM, Kennedy JP, Hopkins CR, Conn PJ, Lindsley CW. Heterobiaryl and heterobiaryl ether derived M5 positive allosteric modulators. Bioorg Med Chem Lett. 2010 Oct 1;20(19):5617-22. doi: 10.1016/j.bmcl.2010.08.042. Epub 2010 Aug 12. PubMed PMID: 20801651; PubMed Central PMCID: PMC3179183.
2: Bridges TM, Phillip Kennedy J, Noetzel MJ, Breininger ML, Gentry PR, Conn PJ, Lindsley CW. Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part II: development of a potent and highly selective M1 PAM. Bioorg Med Chem Lett. 2010 Mar 15;20(6):1972-5. doi: 10.1016/j.bmcl.2010.01.109. Epub 2010 Feb 1. PubMed PMID: 20156687; PubMed Central PMCID: PMC2834874.
3: Bridges TM, Kennedy JP, Cho HP, Breininger ML, Gentry PR, Hopkins CR, Conn PJ, Lindsley CW. Chemical lead optimization of a pan G(q) mAChR M(1), M(3), M(5) positive allosteric modulator (PAM) lead. Part I: Development of the first highly selective M(5) PAM. Bioorg Med Chem Lett. 2010 Jan 15;20(2):558-62. doi: 10.1016/j.bmcl.2009.11.089. Epub 2009 Nov 22. PubMed PMID: 20004578; PubMed Central PMCID: PMC3177601.
4: Bridges TM, Marlo JE, Niswender CM, Jones CK, Jadhav SB, Gentry PR, Plumley HC, Weaver CD, Conn PJ, Lindsley CW. Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins. J Med Chem. 2009 Jun 11;52(11):3445-8. doi: 10.1021/jm900286j. PubMed PMID: 19438238; PubMed Central PMCID: PMC3875304.
Chemical Structure