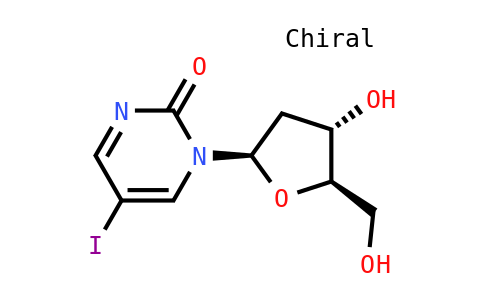
1: Kummar S, Anderson L, Hill K, Majerova E, Allen D, Horneffer Y, Ivy SP, Rubinstein L, Harris P, Doroshow JH, Collins JM. First-in-human phase 0 trial of oral 5-iodo-2-pyrimidinone-2'-deoxyribose in patients with advanced malignancies. Clin Cancer Res. 2013 Apr 1;19(7):1852-7. doi: 10.1158/1078-0432.CCR-12-3118. Epub 2013 Feb 12. PubMed PMID: 23403637; PubMed Central PMCID: PMC3658146.
2: Bihina Bella MA, Eloff JH, Olivier MS. A fraud management system architecture for next-generation networks. Forensic Sci Int. 2009 Mar 10;185(1-3):51-8. doi: 10.1016/j.forsciint.2008.12.013. Epub 2009 Jan 24. PubMed PMID: 19168299.
3: Bose ME, Littrell JC, Patzer AD, Kraft AJ, Metallo JA, Fan J, Henrickson KJ. The Influenza Primer Design Resource: a new tool for translating influenza sequence data into effective diagnostics. Influenza Other Respir Viruses. 2008 Jan;2(1):23-31. doi: 10.1111/j.1750-2659.2007.00031.x. PubMed PMID: 19453490.
4: Kinsella TJ, Kinsella MT, Seo Y, Berk G. 5-iodo-2-pyrimidinone-2'-deoxyribose-mediated cytotoxicity and radiosensitization in U87 human glioblastoma xenografts. Int J Radiat Oncol Biol Phys. 2007 Nov 15;69(4):1254-61. PubMed PMID: 17967315; PubMed Central PMCID: PMC2128756.
5: Saif MW, Berk G, Cheng YC, Kinsella TJ. IPdR: a novel oral radiosensitizer. Expert Opin Investig Drugs. 2007 Sep;16(9):1415-24. Review. PubMed PMID: 17714027.
6: Kinsella TJ, Kinsella MT, Hong S, Johnson JP, Burback B, Tosca PJ. Toxicology and pharmacokinetic study of orally administered 5-iodo-2-pyrimidinone-2'deoxyribose (IPdR) x 28 days in Fischer-344 rats: impact on the initial clinical phase I trial design of IPdR-mediated radiosensitization. Cancer Chemother Pharmacol. 2008 Feb;61(2):323-34. Epub 2007 Jun 12. PubMed PMID: 17562042.
7: Seo Y, Yan T, Schupp JE, Radivoyevitch T, Kinsella TJ. Schedule-dependent drug effects of oral 5-iodo-2-pyrimidinone-2'-deoxyribose as an in vivo radiosensitizer in U251 human glioblastoma xenografts. Clin Cancer Res. 2005 Oct 15;11(20):7499-507. PubMed PMID: 16243824.
8: Seo Y, Yan T, Schupp JE, Colussi V, Taylor KL, Kinsella TJ. Differential radiosensitization in DNA mismatch repair-proficient and -deficient human colon cancer xenografts with 5-iodo-2-pyrimidinone-2'-deoxyribose. Clin Cancer Res. 2004 Nov 15;10(22):7520-8. PubMed PMID: 15569982.
9: Kinsella TJ, Schupp JE, Davis TW, Berry SE, Hwang HS, Warren K, Balis F, Barnett J, Sands H. Preclinical study of the systemic toxicity and pharmacokinetics of 5-iodo-2-deoxypyrimidinone-2'-deoxyribose as a radiosensitizing prodrug in two, non-rodent animal species: implications for phase I study design. Clin Cancer Res. 2000 Sep;6(9):3670-9. PubMed PMID: 10999760.
10: Kinsella TJ, Vielhuber KA, Kunugi KA, Schupp J, Davis TW, Sands H. Preclinical toxicity and efficacy study of a 14-day schedule of oral 5-iodo-2-pyrimidinone-2'-deoxyribose as a prodrug for 5-iodo-2'-deoxyuridine radiosensitization in U251 human glioblastoma xenografts. Clin Cancer Res. 2000 Apr;6(4):1468-75. PubMed PMID: 10778979.
11: Kinsella TJ, Kunugi KA, Vielhuber KA, Potter DM, Fitzsimmons ME, Collins JM. Preclinical evaluation of 5-iodo-2-pyrimidinone-2'-deoxyribose as a prodrug for 5-iodo-2'-deoxyuridine-mediated radiosensitization in mouse and human tissues. Clin Cancer Res. 1998 Jan;4(1):99-109. PubMed PMID: 9516958.
12: Kinsella TJ, Kunugi KA, Vielhuber KA, McCulloch W, Liu SH, Cheng YC. An in vivo comparison of oral 5-iodo-2'-deoxyuridine and 5-iodo-2-pyrimidinone-2'-deoxyribose toxicity, pharmacokinetics, and DNA incorporation in athymic mouse tissues and the human colon cancer xenograft, HCT-116. Cancer Res. 1994 May 15;54(10):2695-700. PubMed PMID: 8168099.
13: Chang CN, Doong SL, Cheng YC. Conversion of 5-iodo-2-pyrimidinone-2'-deoxyribose to 5-iodo-deoxyuridine by aldehyde oxidase. Implication in hepatotropic drug design. Biochem Pharmacol. 1992 May 28;43(10):2269-73. PubMed PMID: 1599512.
14: Lewandowski GA, Cheng YC. Mechanism and mode of action of 5-iodo-2-pyrimidinone 2'-deoxyribonucleoside, a potent anti-herpes simplex virus compound, in herpes simplex virus-infected cells. Mol Pharmacol. 1991 Jan;39(1):27-33. PubMed PMID: 1846219.
15: Efange SM, Alessi EM, Shih HC, Cheng YC, Bardos TJ. Synthesis and biological activities of 2-pyrimidinone nucleosides. 2. 5-Halo-2-pyrimidinone 2'-deoxyribonucleosides. J Med Chem. 1985 Jul;28(7):904-10. PubMed PMID: 2989522.