Linzagolix
Linzagolix is a novel, orally administered GnRH receptor antagonist that potentially provides effective management of endometriosis-associated pain while mitigating bone mineral density loss and other adverse effects typically associated with currently approved treatments.
For research use only. We do not sell to patients.
Chemical Information
| Name | Linzagolix |
|---|---|
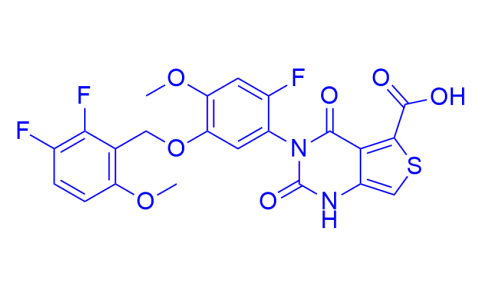
| Iupac Chemical Name | 3-{5-[(2,3-difluoro-6-methoxyphenyl)methoxy]-2-fluoro-4-methoxyphenyl}-2,4-dioxo-1,2,3,4-tetrahydrothieno[3,4-d]pyrimidine-5-carboxylic acid |
| Synonyms | Linzagolix ; OBE2109 ; OBE 2109 ; OBE-2109 ; KLH-2109 ; KLH 2109 ; KLH2109 ; |
| Molecular Formula | C22H15F3N2O7S |
| Molecular Weight | 508.42 |
| Smile | O=C(C1=C(C(N2C3=CC(OCC4=C(OC)C=CC(F)=C4F)=C(OC)C=C3F)=O)C(NC2=O)=CS1)O |
| InChiKey | BMAAMIIYNNPHAB-UHFFFAOYSA-N |
| InChi | InChI=1S/C22H15F3N2O7S/c1-32-14-4-3-10(23)18(25)9(14)7-34-16-6-13(11(24)5-15(16)33-2)27-20(28)17-12(26-22(27)31)8-35-19(17)21(29)30/h3-6,8H,7H2,1-2H3,(H,26,31)(H,29,30) |
| CAS Number | 935283-04-8 |
| Related CAS | 935283-04-8 (free) ; 1321816-57-2 (choline) |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | Off-white solid |
|---|---|
| Purity | ≧98.0% |
| Storage | Dry, dark and at 0-4 ℃ for short term (days to weeks) or -20 ℃ for long term (months to years). |
| Solubility | Soluble in DMSO |
| Handling | Refer to MSDS operation |
| Shipping Condition | Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs. |
| HS Code | 2934200090 |
| Targets | GnRH receptor antagonist |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Borini A, Coticchio G. Gonadotropin-releasing hormone antagonist linzagolix: possible treatment for assisted reproduction patients presenting with adenomyosis and endometriosis? Fertil Steril. 2020 Sep;114(3):517-518. doi: 10.1016/j.fertnstert.2020.06.003. Epub 2020 Aug 3. PMID: 32762949.
2: Donnez O, Donnez J. Gonadotropin-releasing hormone antagonist (linzagolix): a new therapy for uterine adenomyosis. Fertil Steril. 2020 Sep;114(3):640-645. doi: 10.1016/j.fertnstert.2020.04.017. Epub 2020 Jun 2. PMID: 32507315.
3: Donnez J, Taylor HS, Taylor RN, Akin MD, Tatarchuk TF, Wilk K, Gotteland JP, Lecomte V, Bestel E. Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone-antagonist: a randomized clinical trial. Fertil Steril. 2020 Jul;114(1):44-55. doi: 10.1016/j.fertnstert.2020.02.114. Epub 2020 Jun 4. PMID: 32505383.
4: Pohl O, Marchand L, Bell D, Gotteland JP. Effects of combined GnRH receptor antagonist linzagolix and hormonal add-back therapy on vaginal bleeding-delayed add-back onset does not improve bleeding pattern. Reprod Sci. 2020 Apr;27(4):988-995. doi: 10.1007/s43032-020-00172-z. Epub 2020 Feb 25. PMID: 32100275.
Chemical Structure