L006235
L006235 is a potent, reversible cathepsin K inhibitor (IC50 = 0.25 nM) that displays > 4000-fold selectivity over cathepsins B, L and S.
For research use only. We do not sell to patients.
Chemical Information
| Name | L006235 |
|---|---|
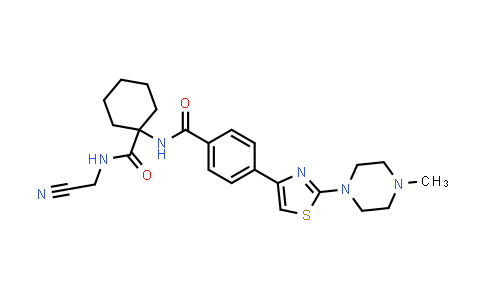
| Iupac Chemical Name | N-(1-((cyanomethyl)carbamoyl)cyclohexyl)-4-(2-(4-methylpiperazin-1-yl)thiazol-4-yl)benzamide |
| Synonyms | L006235; L 006235; L-006235. |
| Molecular Formula | C24H30N6O2S |
| Molecular Weight | 466.6 |
| Smile | O=C(NC1(C(NCC#N)=O)CCCCC1)C2=CC=C(C3=CSC(N4CCN(C)CC4)=N3)C=C2 |
| InChiKey | FIVYCSWOCXEWSE-UHFFFAOYSA-N |
| InChi | InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) |
| CAS Number | 294623-49-7 |
| Related CAS |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | Solid powder |
|---|---|
| Purity | 98% Min. |
| Storage | Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
| Solubility | Soluble in DMSO |
| Handling | |
| Shipping Condition | Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs. |
| HS Code |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Soung do Y, Gentile MA, Duong le T, Drissi H. Effects of pharmacological inhibition of cathepsin K on fracture repair in mice. Bone. 2013 Jul;55(1):248-55. doi: 10.1016/j.bone.2013.02.010. Epub 2013 Feb 26. PubMed PMID: 23486186.
2: Hayami T, Zhuo Y, Wesolowski GA, Pickarski M, Duong le T. Inhibition of cathepsin K reduces cartilage degeneration in the anterior cruciate ligament transection rabbit and murine models of osteoarthritis. Bone. 2012 Jun;50(6):1250-9. doi: 10.1016/j.bone.2012.03.025. Epub 2012 Mar 30. PubMed PMID: 22484689.
3: Pennypacker BL, Duong le T, Cusick TE, Masarachia PJ, Gentile MA, Gauthier JY, Black WC, Scott BB, Samadfam R, Smith SY, Kimmel DB. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res. 2011 Feb;26(2):252-62. doi: 10.1002/jbmr.223. PubMed PMID: 20734451.
4: Svelander L, Erlandsson-Harris H, Astner L, Grabowska U, Klareskog L, Lindstrom E, Hewitt E. Inhibition of cathepsin K reduces bone erosion, cartilage degradation and inflammation evoked by collagen-induced arthritis in mice. Eur J Pharmacol. 2009 Jun 24;613(1-3):155-62. doi: 10.1016/j.ejphar.2009.03.074. Epub 2009 Apr 7. PubMed PMID: 19358841.
5: Le Gall C, Bonnelye E, Clézardin P. Cathepsin K inhibitors as treatment of bone metastasis. Curr Opin Support Palliat Care. 2008 Sep;2(3):218-22. doi: 10.1097/SPC.0b013e32830baea9. Review. PubMed PMID: 18685424.
6: Desmarais S, Black WC, Oballa R, Lamontagne S, Riendeau D, Tawa P, Duong le T, Pickarski M, Percival MD. Effect of cathepsin k inhibitor basicity on in vivo off-target activities. Mol Pharmacol. 2008 Jan;73(1):147-56. Epub 2007 Oct 16. PubMed PMID: 17940194.
7: Black WC, Percival MD. The consequences of lysosomotropism on the design of selective cathepsin K inhibitors. Chembiochem. 2006 Oct;7(10):1525-35. Review. PubMed PMID: 16921579.
8: Falgueyret JP, Desmarais S, Oballa R, Black WC, Cromlish W, Khougaz K, Lamontagne S, Massé F, Riendeau D, Toulmond S, Percival MD. Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem. 2005 Dec 1;48(24):7535-43. PubMed PMID: 16302795.
9: Palmer JT, Bryant C, Wang DX, Davis DE, Setti EL, Rydzewski RM, Venkatraman S, Tian ZQ, Burrill LC, Mendonca RV, Springman E, McCarter J, Chung T, Cheung H, Janc JW, McGrath M, Somoza JR, Enriquez P, Yu ZW, Strickley RM, Liu L, Venuti MC, Percival MD, Falgueyret JP, Prasit P, Oballa R, Riendeau D, Young RN, Wesolowski G, Rodan SB, Johnson C, Kimmel DB, Rodan G. Design and synthesis of tri-ring P3 benzamide-containing aminonitriles as potent, selective, orally effective inhibitors of cathepsin K. J Med Chem. 2005 Dec 1;48(24):7520-34. PubMed PMID: 16302794.
Chemical Structure