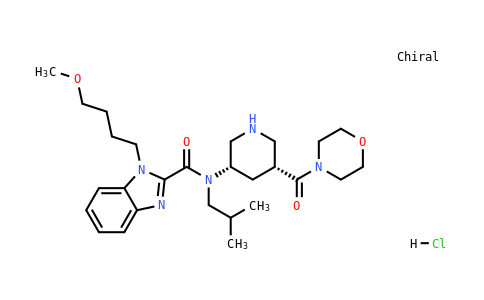
Imarikiren HCl
Imarikiren, also known as TAK-272, is a potent, selective and orally active direct renin inhibitor for the treatment of diabetic nephropathies. TAK-272 showed potent inhibitory activity against human renin (IC50 = 2.1 nM) in hPRA assay and excellent selectivity against other aspartic proteases, such as pepsin (IC50 > 10 μM) and cathepsin D (IC50 > 10 μM). TAK-272 exhibited a dramatically improved PK profile in rats (F = 25.2%). TAK-272 (3 and 10 mg/kg, p.o.) exhibited potent and long-lasting antihypertensive efficacy in a dose-dependent manner. TAK-272 HCl is currently undergoing human clinical trials.
For research use only. We do not sell to patients.
Chemical Information
| Name | Imarikiren HCl |
|---|---|
| Iupac Chemical Name | 1-(4-methoxybutyl)-N-(2-methylpropyl)-N-[(3S,5R)-5-(morpholin-4-yl)carbonylpiperidin-3-yl]-1H-benzimidazole-2-carboxamide hydrochloride |
| Synonyms | TAK-272; TAK 272; TAK272; TAK-272 hydrochloride; TAK-272 HCl. Imarikiren HCl | |
| Molecular Formula | C27H42ClN5O4 |
| Molecular Weight | 536.11 |
| Smile | O=C(C1=NC2=CC=CC=C2N1CCCCOC)N(CC(C)C)[C@@H]3CNC[C@H](C(N4CCOCC4)=O)C3.[H]Cl |
| InChiKey | PUXOYQIZZIWCHH-NSLUPJTDSA-N |
| InChi | InChI=1S/C27H41N5O4.ClH/c1-20(2)19-32(22-16-21(17-28-18-22)26(33)30-11-14-36-15-12-30)27(34)25-29-23-8-4-5-9-24(23)31(25)10-6-7-13-35-3;/h4-5,8-9,20-22,28H,6-7,10-19H2,1-3H3;1H/t21-,22+;/m1./s1 |
| CAS Number | 1202269-24-6 |
| Related CAS |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | Off-white solid |
|---|---|
| Purity | 98% Min. |
| Storage | Dry, dark and at 0 - 4℃ for short term (days to weeks) or -20℃ for long term (months to years). |
| Solubility | Soluble in DMSO |
| Handling | |
| Shipping Condition | Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs. |
| HS Code |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
Discovery of TAK-272: A Novel, Potent, and Orally Active Renin Inhibitor Yasuhiro Imaeda, Hidekazu Tokuhara, Yoshiyuki Fukase, Ray Kanagawa, Yumiko Kajimoto, Keiji Kusumoto, Mitsuyo Kondo, Gyorgy Snell, Craig A. Behnke, and Takanobu Kuroita Publication Date (Web): September 12, 2016 (Letter) DOI: 10.1021/acsmedchemlett.6b00251
Chemical Structure