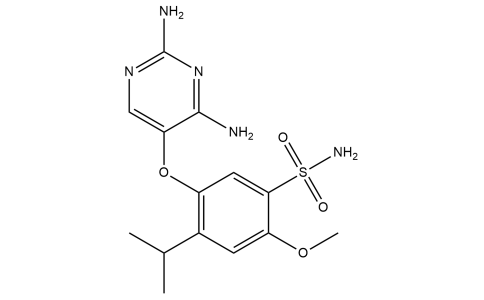
Gefapixant
Gefapixant, also known as AF-219 or MK-7264, is a P2X3 receptor antagonist. Gefapixant is currently under clinical trials for chronic cough. It is believed that excessive activation of P2X3 receptors is associated with hyper-sensitization of sensory neurons. Neuronal hyper-sensitization in the airways and lungs, triggered by injury or infection, can cause an exaggerated, persistent and frequent urge to cough, so called chronic cough. P2X3 receptors seem to have a key role in mediation of cough neuronal hypersensitivity. Antagonists of P2X3 receptors such as AF-219 are a promising new group of antitussives.
For research use only. We do not sell to patients.
Chemical Information
| Name | Gefapixant |
|---|---|
| Iupac Chemical Name | 5-[(2,4-diaminopyrimidin-5-yl)oxy]-2-methoxy-4-(propan-2-yl)benzene-1-sulfonamide |
| Synonyms | Gefapixant ; MK-7264 ; MK 7264 ; MK7264 ; AF-219 ; AF219 ; AF 219 ; RO4926219 ; RO-4926219 ; RO 4926219 ; |
| Molecular Formula | C14H19N5O4S |
| Molecular Weight | 353.39 |
| Smile | O=S(C1=CC(OC2=CN=C(N)N=C2N)=C(C(C)C)C=C1OC)(N)=O |
| InChiKey | HLWURFKMDLAKOD-UHFFFAOYSA-N |
| InChi | InChI=1S/C14H19N5O4S/c1-7(2)8-4-10(22-3)12(24(17,20)21)5-9(8)23-11-6-18-14(16)19-13(11)15/h4-7H,1-3H3,(H2,17,20,21)(H4,15,16,18,19) |
| CAS Number | 1015787-98-0 |
| Related CAS | 1015787-98-0 |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | Off-white solid to white solid |
|---|---|
| Purity | 98% |
| Storage | Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
| Solubility | Soluble in DMSO |
| Handling | Avoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation. |
| Shipping Condition | Shipped under ambient temperature |
| HS Code |
| Targets | P2X3 Antagonist |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Wang J, Wang Y, Cui WW, Huang Y, Yang Y, Liu Y, Zhao WS, Cheng XY, Sun WS, Cao P, Zhu MX, Wang R, Hattori M, Yu Y. Druggable negative allosteric site of P2X3 receptors. Proc Natl Acad Sci U S A. 2018 May 8;115(19):4939-4944. doi: 10.1073/pnas.1800907115. Epub 2018 Apr 19. PubMed PMID: 29674445; PubMed Central PMCID: PMC5948998.
2: Burnstock G. The therapeutic potential of purinergic signalling. Biochem Pharmacol. 2018 May;151:157-165. doi: 10.1016/j.bcp.2017.07.016. Epub 2017 Jul 21. Review. PubMed PMID: 28735873.
3: Sheridan C. Merck stakes out 'irritable' neuron territory with $1.25 billion. Nat Biotechnol. 2016 Sep 8;34(9):900. doi: 10.1038/nbt0916-900. PubMed PMID: 27606448.
4: Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, Smith JA. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015 Mar 28;385(9974):1198-205. doi: 10.1016/S0140-6736(14)61255-1. Epub 2014 Nov 25. PubMed PMID: 25467586.
5: Morice AH. Developing antitussives the clinician's pipeline-what do we need? J Thorac Dis. 2014 Oct;6(Suppl 7):S735-8. doi: 10.3978/j.issn.2072-1439.2014.08.40. Review. PubMed PMID: 25383208; PubMed Central PMCID: PMC4222922.
6: Ochoa-Cortes F, Liñán-Rico A, Jacobson KA, Christofi FL. Potential for developing purinergic drugs for gastrointestinal diseases. Inflamm Bowel Dis. 2014 Jul;20(7):1259-87. doi: 10.1097/MIB.0000000000000047. Review. PubMed PMID: 24859298; PubMed Central PMCID: PMC4340257.
7: Ford AP, Undem BJ. The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci. 2013 Dec 19;7:267. doi: 10.3389/fncel.2013.00267. Review. PubMed PMID: 24391544; PubMed Central PMCID: PMC3867694.
Chemical Structure