Flavopiridol ( Alvocidib )
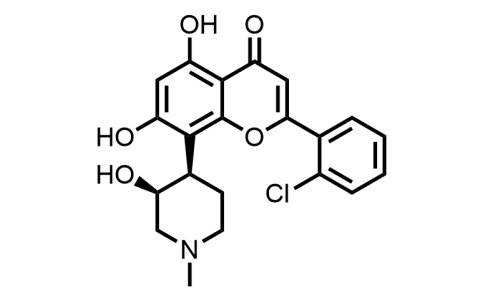
Alvocidib is a synthetic dihydroxyflavone that is 5,7-dihydroxyflavone which is substituted by a 3-hydroxy-1-methylpiperidin-4-yl group at position 8 and by a chlorine at the 2' position (the (-)-3S,4R stereoisomer). A cyclin-dependent kinase 9 (CDK9) inhibitor, it has been studied for the treatment of acute myeloid leukaemia, arthritis and atherosclerotic plaque formation. It has a role as an antineoplastic agent, an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor, an antirheumatic drug and an apoptosis inducer. It is a dihydroxyflavone, a hydroxypiperidine, a member of monochlorobenzenes and a tertiary amino compound. It is a conjugate base of an alvocidib(1+).
For research use only. We do not sell to patients.
Chemical Information
| Name | Flavopiridol ( Alvocidib ) |
|---|---|
| Iupac Chemical Name | 2-(2-chlorophenyl)-5,7-dihydroxy-8-((3S,4R)-3-hydroxy-1-methylpiperidin-4-yl)-4H-chromen-4-one |
| Synonyms | L 86-8275 ; L-868275 ; L 868275 ; L868275 ; HMR 1274 ; HMR-1274 ; HMR1274 ; Flavoperidol ; Alvocidib ; |
| Molecular Formula | C21H20ClNO5 |
| Molecular Weight | 401.84 |
| Smile | O=C1C=C(C2=CC=CC=C2Cl)OC3=C([C@@H]4[C@H](O)CN(C)CC4)C(O)=CC(O)=C13 |
| InChiKey | BIIVYFLTOXDAOV-YVEFUNNKSA-N |
| InChi | InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12-,17+/m0/s1 |
| CAS Number | 146426-40-6 |
| Related CAS | 146426-40-6 |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | Yellow solid |
|---|---|
| Purity | ≧98.0% |
| Storage | Dry, dark and at 0 - 4℃ for short term (days to weeks) or -20℃ for long term (months to years). |
| Solubility | Soluble in DMSO |
| Handling | Refer to MSDS |
| Shipping Condition | Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs. |
| HS Code | 2934200090 |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
Chemical Structure