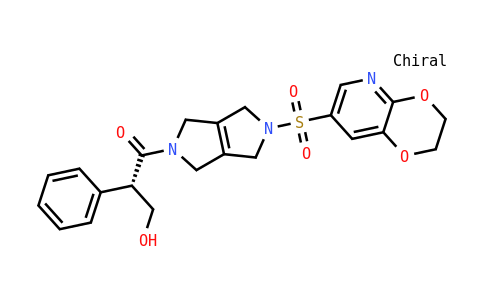
Etavopivat
Etavopivat, also known as FT-4202, is a pyruvate kinase activator. Etavopivat decreased 2,3-diphosphoglycerate in red blood cells (RBCs) from nonhuman primates and healthy subjects and significantly increased hemoglobin (Hb)-oxygen affinity in healthy subjects. Using ex vivo RBCs from donors with sickle cell disease (SCD) (homozygous hemoglobin S or hemoglobin S and C genotype), etavopivat increased Hb-oxygen affinity and reduced sickling under deoxygenation. Etavopivat shows promise as a treatment for SCD that could potentially reduce vaso-occlusion and improve anemia.
For research use only. We do not sell to patients.
Chemical Information
| Name | Etavopivat |
|---|---|
| Iupac Chemical Name | (2S)-1-[5-(2,3-dihydro[1,4]dioxino[2,3-b]pyridine-7-sulfonyl)-3,4,5,6-tetrahydropyrrolo[3,4-c]pyrrol-2(1H)-yl]-3-hydroxy-2-phenylpropan-1-one |
| Synonyms | Etavopivat; FT-4202; FT 4202; FT4202; |
| Molecular Formula | C22H23N3O6S |
| Molecular Weight | 457.50 |
| Smile | O=C(N1CC(CN(S(=O)(C2=CN=C(OCCO3)C3=C2)=O)C4)=C4C1)[C@@H](C5=CC=CC=C5)CO |
| InChiKey | KZFFYEPYCVDOGE-LJQANCHMSA-N |
| InChi | InChI=1S/C22H23N3O6S/c26-14-19(15-4-2-1-3-5-15)22(27)24-10-16-12-25(13-17(16)11-24)32(28,29)18-8-20-21(23-9-18)31-7-6-30-20/h1-5,8-9,19,26H,6-7,10-14H2/t19-/m1/s1 |
| CAS Number | 2245053-57-8 |
| Related CAS |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | Off-white solid |
|---|---|
| Purity | 98% Min. |
| Storage | Dry, dark and at 0 - 4℃ for short term (days to weeks) or -20℃ for long term (months to years). |
| Solubility | Soluble in DMSO |
| Handling | |
| Shipping Condition | Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs. |
| HS Code |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Schroeder P, Fulzele K, Forsyth S, Ribadeneira MD, Guichard S, Wilker E, Marshall CG, Drake A, Fessler R, Konstantinidis DG, Seu KG, Kalfa TA. Etavopivat, a Pyruvate Kinase Activator in Red Blood Cells, for the Treatment of Sickle Cell Disease. J Pharmacol Exp Ther. 2022 Mar;380(3):210-219. doi: 10.1124/jpet.121.000743. Epub 2022 Jan 14. PMID: 35031585.
2: Fattizzo B, Motta I. Rise of the planet of rare anemias: An update on emerging treatment strategies. Front Med (Lausanne). 2023 Jan 9;9:1097426. doi: 10.3389/fmed.2022.1097426. PMID: 36698833; PMCID: PMC9868867.
3: Forsyth S, Schroeder P, Geib J, Vrishabhendra L, Konstantinidis DG, LaSalvia K, Ribadeneira MD, Wu E, Kelly P, Kalfa TA. Safety, Pharmacokinetics, and Pharmacodynamics of Etavopivat (FT-4202), an Allosteric Activator of Pyruvate Kinase-R, in Healthy Adults: A Randomized, Placebo-Controlled, Double-Blind, First-in-Human Phase 1 Trial. Clin Pharmacol Drug Dev. 2022 May;11(5):654-665. doi: 10.1002/cpdd.1058. Epub 2022 Jan 12. PMID: 35019238; PMCID: PMC9306898.
4: Matte A, Federti E, De Franceschi L. Erythrocyte pyruvate kinase activation in red cell disorders. Curr Opin Hematol. 2023 May 1;30(3):93-98. doi: 10.1097/MOH.0000000000000758. Epub 2023 Feb 9. PMID: 36853806.
5: Chou ST, Hendrickson JE, Fasano RM. Transfusion therapy for sickle cell disease: what's new? Blood Adv. 2023 Jun 13;7(11):2551-2553. doi: 10.1182/bloodadvances.2022009283. PMID: 36562748; PMCID: PMC10242629.
Chemical Structure