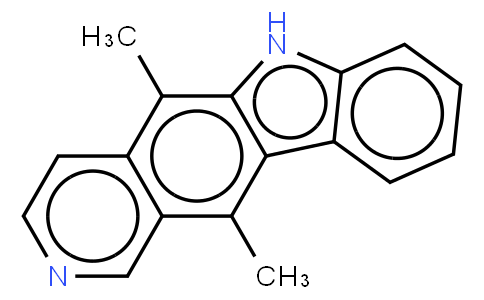
Ellipticine
Catalog No: 16122811
CAS Number: 519-23-3
Purity: 98%
Ellipticine is a DNA intercalating agent and a DNA topoisomerase II inhibitor. Ellipticine is also a natural product, isolated in 1959 from the Australian evergreen tree of the Apocynaceae family. Ellipticine was found to be an extremely promising anticancer drug. The planar polycyclic structure was found to interact with DNA through intercalation, exhibiting a high DNA binding affinity (10(6) M(-1)). The presence of protonatable ring nitrogens distinguished ellipticine from other simple intercalators. Both monocationic and uncharged species were found to be present under physiological conditions. The positive charge stabilized the binding of ellipticine to nucleic acids, while the more lipophilic uncharged compound was shown to readily penetrate membrane barriers. The structural nature of these compounds offers a plausible basis for the implication of multiple modes of action, including DNA binding, interactions with membrane barriers, oxidative bioactivation and modification of enzyme function; most notably that of topoisomerase II and telomerase. ( Curr Med Chem Anticancer Agents. 2004 Mar;4(2):149-72 ).
For research use only. We do not sell to patients.
Chemical Information
| Name | Ellipticine |
|---|---|
| Iupac Chemical Name | Ellipticine |
| Synonyms | CCG-36483; CCG36483; CCG 36483; BRN-0221300; BRN0221300; BRN 0221300; DB-052047; K00071; LP00531; LS-133282; NSC 71795; NSC71795; NSC-71795; |
| Molecular Formula | C17H14N2 |
| Molecular Weight | 246.31 |
| Smile | Cc1c2ccncc2c(c3c1[nH]c4c3cccc4)C |
| InChiKey | CTSPAMFJBXKSOY-UHFFFAOYSA-N |
| InChi | InChI=1S/C17H14N2/c1-10-14-9-18-8-7-12(14)11(2)17-16(10)13-5-3-4-6-15(13)19-17/h3-9,19H,1-2H3 |
| CAS Number | 519-23-3 |
| MDL | MFCD00010524 |
| Related CAS |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | crystalline solid |
|---|---|
| Purity | 98% |
| Storage | 3 years -20ºCpowder |
| Solubility | Soluble in DMSO |
| Handling | |
| Shipping Condition | Shipped under ambient temperature as non-hazardous chemical. |
| HS Code |
Coming soon.
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
Not available
Chemical Structure